Ultrasound dosage in sports / Rojas-Valverde y cols. ISSN 2215-5562. Rev.Ter. Julio-Diciembre de 2021; Vol. 15 N°2: 7-21
ARTÍCULO DE REVISION
Methodological considerations when dosing therapeutic ultrasound in sports: a narrative review
Consideraciones metodológicas a la hora de dosificar el ultrasonido terapéutico en el deporte: una revisión narrativa
Título corto: Ultrasound dosage in sports
Autores: Daniel Rojas-Valverde1, Luis D. Rojas-Valverde2, Andrea Fallas Campos3, Braulio Sánchez Ureña3, Randall Gutiérrez-Vargas1
Filiación: 1Centro de Investigación en Salud y Deporte (CIDISAD), Escuela Ciencias del Movimiento Humano y Calidad de Vida (CIEMHCAVI), Universidad Nacional, Heredia, Costa Rica. 2 Caja Costarricense del Seguro Social (CCSS), San José, Costa Rica. 3 Programa de Ciencias del Ejercicio y la Salud (PROCESA), Escuela Ciencias del Movimiento Humano y Calidad de Vida (CIEMHCAVI), Universidad Nacional, Heredia, Costa Rica.
Correspondencia: Daniel Rojas-Valverde. Correo electrónico: drojasv@hotmail.com
Forma de citar: Rojas Valverde D, Rojas Valverde JD, Fallas Campos A, Sánchez Ureña B, Gutiérrez Vargas R. Methodological considerations when dosing therapeutic ultrasound in sports: a narrative review. Rev Ter. 2021;15(2): 7-21
Financiamiento: Ninguno
Fecha de envío: 10 de diciembre del 2020
Fecha de aceptación: 22 de marzo del 2021
Declaración de conflictos de Interés: Ninguno
Abbreviatures: Therapeutic ultrasound, TUS.
Abstract
The use of therapeutic ultrasound (TUS) in sports science, medicine and rehabilitation has increased significantly in the last decade. This growth has been accompanied by a great scientific interest for the study of responses during injury treatment and the finding of optimal dosage. Therefore, the purpose of this narrative review was to explore the knowledge reported to date on the use of TUS and the methodological considerations when applying it in sport injury assessment. An electronic search (PubMed [MEDLINE], Science Direct [EMBASE], Web of Science [WoS], and Google Scholar) was conducted following systematic review guidelines (PRISMA) and including only systematic reviews about TUS application in sports. The result of this review is the presentation of some methodological considerations when using TUS in sport practice, giving a general guidance for it safety use, efficiency and effectiveness considering the need to rapidly recover from injuries in sports.
Keywords: Injury intervention, return to play, evidence-based physical therapy
Resumen
El uso de la ecografía terapéutica (TUS) en la ciencia del deporte, la medicina y la rehabilitación ha aumentado significativamente en la última década. Este crecimiento ha estado acompañado de un gran interés científico por el estudio de las respuestas durante el tratamiento de lesiones y el hallazgo de la dosis óptima. Por lo tanto, el propósito de esta revisión narrativa fue explorar el conocimiento informado hasta la fecha sobre el uso de TUS y las consideraciones metodológicas al aplicarlo en la evaluación de lesiones deportivas. Se realizó una búsqueda electrónica (PubMed [MEDLINE], Science Direct [EMBASE], Web of Science [WoS] y Google Scholar) siguiendo las pautas de revisión sistemática (PRISMA), e incluyó solo revisiones sistemáticas sobre la aplicación de TUS en los deportes. El resultado de esta revisión es la presentación de algunas consideraciones metodológicas al utilizar la TUS en la práctica deportiva, dando una orientación general para su uso de seguridad, eficiencia y efectividad considerando la necesidad de recuperarse rápidamente de las lesiones en el deporte.
Palabras clave: Intervención en lesiones, regreso al juego, fisioterapia basada en evidencia.
Introduction
Currently elite sports players are prone to congested fixtures with a series of matches or tournaments in a few days1,2. The dynamics of elite sport currently requires the performance of frequent high intensity efforts, recent studies have called this type of effort congested fixture periods and can mean a series of matches or training sessions carried out in a short space of time. These efforts are characterized by having loads of very high intensity and volume. Competing up to three times a week during some periods of the season is very common in professional sports, and sometimes there are only three to four recovery days between these constant matches, which are insufficient to restore the normal homeostasis3,4. Therefore, repetitive competition combined with a short recovery time can cause acute and chronic fatigue, leading to impaired performance and possible injuries. Therefore, it is essential to intervene effectively and efficiently in the recovery of any type of functional and structural alteration.
In the last decade, the sport has experienced accelerated growth and evolution in technological developments, and this is impacting the daily work in the area of sports sciences from researchers to practitioners. This development has allowed the creation of new and specific tools to be used in sport science and medicine, understood as safer, less invasive, and with high validity and reliability5,6. This is fundamental for the monitoring and control of the therapeutic treatment of injuries7,8. In this sense the ultrasound technology has certain advantages in the treatment of sport injuries.
Therapeutic ultrasound (TUS) has been use for more than 80 years as a technological tool for the recovery of musculoskeletal injuries. The ultrasound is a form of mechanical energy and uses mechanical vibration at high frequencies; this is also known as sound energy9,10. The human sound range is from 16Hz to almost 20000Hz. Beyond this frequency the mechanical vibration is known as ultrasound, therefore TUS frequencies typically ranged between 1 tot 3 MHz (1MHz = 1 million pulses per second) and wavelength usually ranged from 1.5mm (1MHz) to 0.5mm (3MHz), the velocity (m/s-1 at which the wave travels through the medium) is approximately 1500m/s-1. Despite its mechanism is not electrical at all it is commonly grouped into the Electro Physical Agents. High frequency TUS is considered at 0.5-10 MHz with intensities up to 1500W/cm2 while low frequency TUS using low power is described as at 20-120 kHz and 0.05-1.0 W/cm2 11.
There are two types of TUS, thermal and mechanical; the principal difference between both of them lay on the rate at which the sound waves penetrate the tissues, but both effects are not separable 12 . Sounds waves are longitudinal waves composed of areas of compression and rarefaction. As the sound waves pass through a tissue cause oscillation generating molecular vibration and friction in the area leading to heat generation (thermal ultrasound therapy); so it can be used to provoke thermal changes, though actual usage in therapy does not focus on this effect but in the vibration of the body area leading to non-thermal effects (mechanical ultrasound therapy)9,12,13.
The relative absorption of the tissues is critical in terms of clinical decision making; the best absorbing material are those with high collagen content (e.g. tendon, fascia, ligament, joint capsule). This means that the application of TUS in a highly absorbing material results in a more effective treatment results13,14.
Considering that there are several factors that could influence the effectiveness and efficiency of TUS as known: wave frequency, wavelength, absorption, reflection and attenuation rate of the tissues, pulsation, impedance of the tissue among others; the therapeutic decisions must be analyzed considering the characteristics of the injury as mechanism, clinic, temporality, severity, and others in order to take the best decisions. Therefore, it is needed to explore the optimal settings in the use of TUS. Therefore, the aim of this review was to explore and systematize the recent knowledge around the use of TUS in the approach of sport injuries.
Methods
A systematic review was performed following the Preferred Reporting Guidelines for Systematic Reviews and Meta-analyzes (PRISMA)15,16. Two authors independently review manuscripts based on risk-of-bias. This assessment was made using a 4-point scale ranging from low to high risk-of-bias qualification and discrepancies between authors were resolved using consensus. The internal quality of each study was assessed using the Office of Health Assessment and Translation (OHAT) Risk of Bias Rating Tool 17.
Data Sources
A literature electronic search was performed at four different databases: PubMed (MEDLINE), Science Direct (EMBASE), Web of Science (WoS) and Google Scholar. The Boolean phrases used as search descriptors were: ¨therapeutic ultrasound AND review AND sports¨. All references were extracted and imported into an open-source research tool (5.0.64, Zotero, USA) to systematize studies.
Data Selection
The following inclusion criteria was considered: studies containing keywords in title or abstract, experimental designs in humans, studies published from 2000 to 2020, studies exploring the effects on injury treatment when using TUS. S tudies initially written in Spanish or English language and systematic review and meta-analyses were included. Studies related to sport-related injuries treatment were included.
Data collection and extraction
Specific exclusion criteria were used to discard studies with low quality or irrelevant to the primary purpose of this narrative review as duplicates, language limitations, studies in animals, full text not available, no systematic reviews or meta analyses, different evaluation methods or technologies, book chapters, abstracts, and articles with severe lack of information. Some critical reviews and fundamental literature were used to back up the evidence found and in order to clarify the findings (see figure 1).
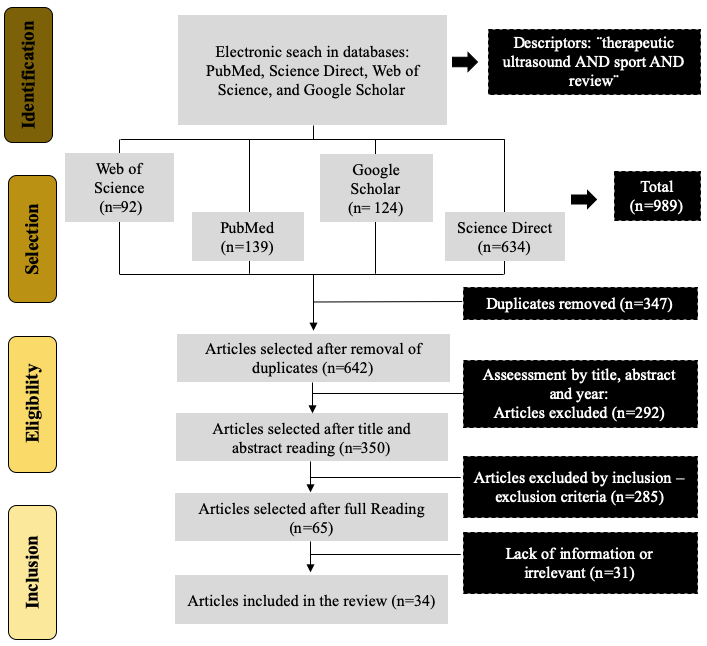
Figure 1. Identification and selection flowchart.
Source: Author´s own preparation
Results
Basic therapeutic principles and considerations
When TUS is employed at an appropriate treatment dose considering optimal treatment parameter such as intensity, pulsing and time, the benefits lay into the promotion effect on the whole healing cascade. That’s why some methodological consideration must be taken into account when dosing TUS in sport-related injuries.
Frequency: the tissue depth must lead the decision on the frequency, pulse ratio and intensity used during the treatment. For example, 3MHz could be absorbed more rapidly in the tissues and it must be considered more appropriate for superficial injuries, while 1MHz energy is absorbed less rapidly and can be more effective in deeper tissues. There is a consensus on the use of 2cm as the boundary between deep and superficial lesions when choosing therapeutic ultrasound as treatment method (see figure 2). When heating rate is analyzed, 1MHz could generate 0.2ºC per W/cm2, per min; besides 3MHz is a fast heater with 0.6ºC per W/cm2, per min. This must be considered due to the suggested theory that an increase of 1°C over baseline muscle temperature of 36°C to 37°C accelerates the metabolic rate in tissue. An increase of 2°C to 3°C reduces muscle spasm, pain, and chronic inflammation and increases blood flow 18 . The use of 1MHz usually lead to an increase in tissue temperature up to 40ºC and 3MHz to 42ºC 19,20.
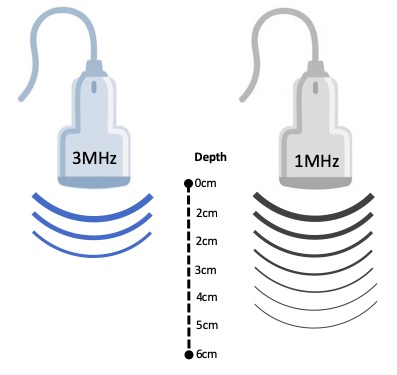
Figure 2. Representation of the 1 and 3MHz depth tissue penetration.
Source: Author´s own preparation
Thermal vs non thermal effects: the non-thermal effects of TUS are mainly attributed to a combination of physical and mechanical effects as cavitation and acoustic streaming12,13. Some evidence points out the effect of micro massage but it needs future confirmation. Cavitation must be understood as formation of gas filled voids within the tissues and fluids; there are two kind of cavitation the stable and the unstable that may produce different effects. The stable cavitation is used for therapeutic purposes considering the unstable made gas bubbles that may collapse generating a large amount of energy that is detrimental to tissue viability. The stable cavitation relays on the formation and growth of gas bubbles by accumulation of gas, it is needed 1000 cycles (waves) to reach maximum size, and this enhances the acoustic streaming phenomena. This acoustic streaming is defined as a small scale eddying of fluids near a vibrating structure such cell membranes and the membrane of the bubbles resulted from cavitation 21 (see figure 3).
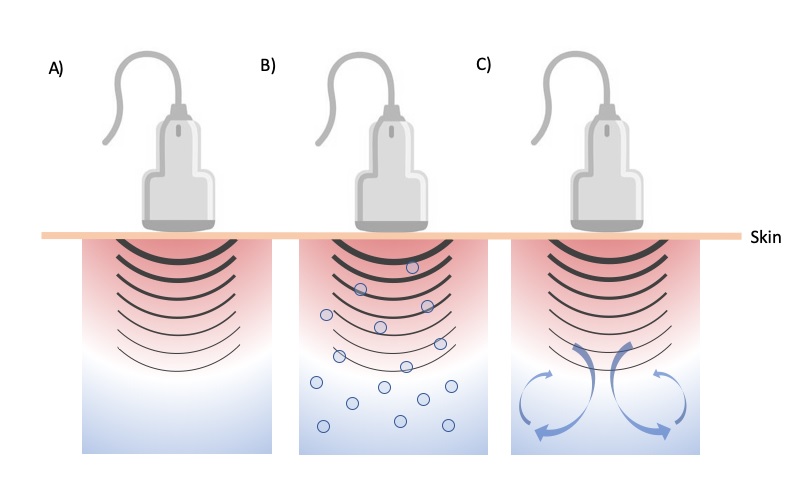
Figure 3. Representation of A) progressive heating by dissipation of the acoustic energy, B) formation, growth, and collapse of gas bubbles (cavitation) and C) fluid flow and possible formation of convection cells (acoustic streaming)
Source: Author´s own preparation
This acoustic streaming use affects cells diffusion rates and membrane permeability, there are also changes in sodium ion permeability, so the cell membrane potential is also altered. Calcium ion transport is modified affecting enzyme control mechanisms especially those concerning cellular secretion and protein synthesis. The combination of stable cavitation and acoustic streaming is the cause of cell membrane excitation that result in the increase of whole cell activity levels, the real responsible effect of TUS22,23.
So this review made substantial contribution to understand how the combination of cavitation and acoustic streaming could lead to tissue repair considering a complex series of cascaded events; provoking significant stimulating effects on critical process to repair tissues and generate scar tissue as inflammation, proliferation and remodeling 24–26 . First, during the inflammatory phase TUS have a pro-inflammatory influence (inflammatory optimizer) rather than anti-inflammatory stimulating platelets, phagocytic white cells, macrophages and mast cells. The application of this therapeutic method induces degranulation of mast cells causing the release of inflammatory mediators (e.g., prostaglandins and leukotreine) 27-29.
During the proliferative phase, also known as scar tissue production process, TUS has a cell stimulative effect, promoting the activation of fibroblast, endothelial cells and myofibroblast function29, 30. This therapeutic method acts as a pro-proliferative agent in this phase, maximizing the efficiency of the process. In this sense, it has been demonstrated that TUS also increases protein synthesis and enhances fibroplasia and collagen synthesis31 . It is also known that this method could boost the angiogenesis 32. Finally, in the remodeling process TUS helps the scar to adopts functional characteristics of the tissue that it is repairing 33,34. Mainly, there is an effect on the orientation of the collagen fibers in the scar developing and changing type III collagen to type I collagen. These effects should result in an increase of tensile strength and scar mobility35-37.
Finally, some recommendations in the use of TSU have been made depending on the tissue temperature increase. Non-thermal effects are usually applied to acute injury and tissue healing, mid thermal effects (1ºC) for sub-acute injury and tissue healing, moderate thermal effects (2-3ºC) are used for chronic inflammation, pain and trigger points treatment, and vigorous heating (+4ºC) is performed with the purpose of stretching collagen 38. This is fundamental when choosing the intensity and frequency of the TSU dose. Heating rates may depend on frequency and intensity as follows: an intensity of 0.5 W/cm2, 1 W/cm2, 1.5 W/cm2 and 2 W/cm2 may lead to a 0.04ºC, 0.2ºC, 0.3ºC and 0.4ºC when applying continuous 1MHz and 0.3ºC, 0.6ºC, 0.9ºC and 1.4ºC respectively when using 3MHz39.
Absorption and attenuation : there are energy levels not sufficient to produce a therapeutic effect. This could be cause due to the absorbed energy by the tissues when ultrasound beam penetrates the different tissue layers. So, more energy is absorbed in the superficial tissues than in the deep ones. It must be understood that it is difficult to know the thickness of each tissue layers in an individual patient, so it is well known that 3MHz could penetrate at 2cm and 1MHz at 4cm (50% of energy absorption at this level).
Impedance and coupling medium: it is necessary to remember that tissues present an impedance to the passage of the ultrasound waves, defined by its density and elasticity. Considering the difference between the impedance of the generator and tissues, it is needed a coupling medium, in this case gel-based media seems to be ideal40. Otherwise, there would be no energy transfer, the greater the difference in impedance the greater the reflection of energy. The addition of some active agents as anti-inflammatory drugs and other substances as cortisol or lidocaine to the gel is widely used but needs more research to confirm the real effects, this method is called sonophoresis or phonophoresis and usually is performed using 1-3MHz, 1-2 W/cm2, during 5-10min continuous or pulsed mode 41. Some evidence suggests strong aseptic protocols to avoid bacterial contamination between patients and prevent some tissue damage due to individual tolerance to substances.
Critical application angle: additionally to the differences in impedance, there could be some refractions if the wave does not strike the surface with an angle of 90º, perpendicular to the skin (see figure 3): if not possible positions where the treatment head tiled above 15º should be avoided.
Pulsation: TUS could be applied in pulsed mode with 2ms and 2ms off period (1:1); but this off period could be changed in actual equipment (e.g., 1:2, 1:3, 1:4) allowing greater rest periods (e.g., 4ms, 6ms and 8ms) (see figure 4). This type of output is usually well accepted in the approach of acute lesions. The use of higher pulsations (1:4, 20%) is commonly accepted when treating acute lesions compare to those most chronic that need a continuous beam output. Based on this principle, 1:4 or 1:3 are used for acute injuries, 1:2 and 1:1 for sub-acute and 1:1 or continuous for chronic injuries. The ratio between pulse and rest is defined as pulse output expressed in duty cycle presented in percentage (%), calculated using the following formula:
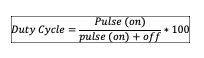
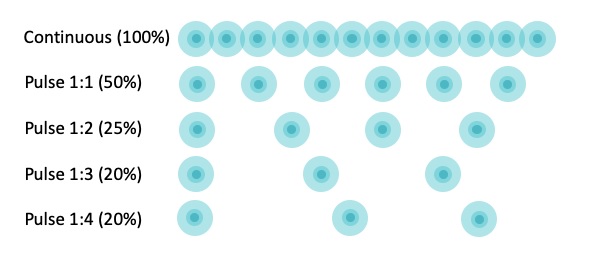
Figure 4. Representation of the pulsation ratio with pulse and off periods
Source: Author´s own preparation
Intensity: the decision when choosing intensity lays on the tissue state. The more acute the lesion, le less intensity is needed to achieve tissue excitement, in the other hand the more chronic the tissue state, the less sensitive and hence, higher intensity is required to provoke a physiological response. Acute 0.1 to 0.3 W/cm2, sub-acute 0.2 to 0.5 W/cm2 and chronic injuries may require 0.5 to 1W/cm2. New evidence has suggests the use of 1.5 up to 2W/cm2 19 . In addition, the injury depth could incidence on the decision of which intensity may be selected. Considering that there is energy absorption when the wave travels through the tissues; it is fundamental to estimate the intensity considering the tissue depth and the frequency of the wave. For these purposes, the figure 5 was made to estimate the real settings to achieve the intensity require at the lesion.
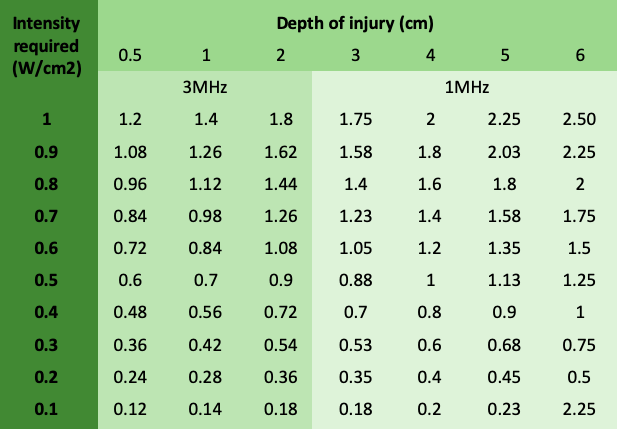
Figure 5. Dosage of therapeutic ultrasound considering depth of injury, intensity required and frequency based on Watson et al. 9,13
Source: Author´s own preparation
Application time: it is commonly considered that the greater the size of the injury, the longer the duration of the ultrasound application. The most common method to estimate the time is to measure how many times the ultrasound treatment head can be placed over the target area. The final intention is to apply 1 min of energy per treatment head area covered. It must be calculated following this formula:

Where Φ= n of head fitting over the lesion area, and Ψ= the sum of the two components of the pulse ratio (ratio 1:4= 5).
Dose calculation : in order to calculate the dosage when applying therapeutic ultrasound, figure 6 could be used to guide the applicant in the practice.
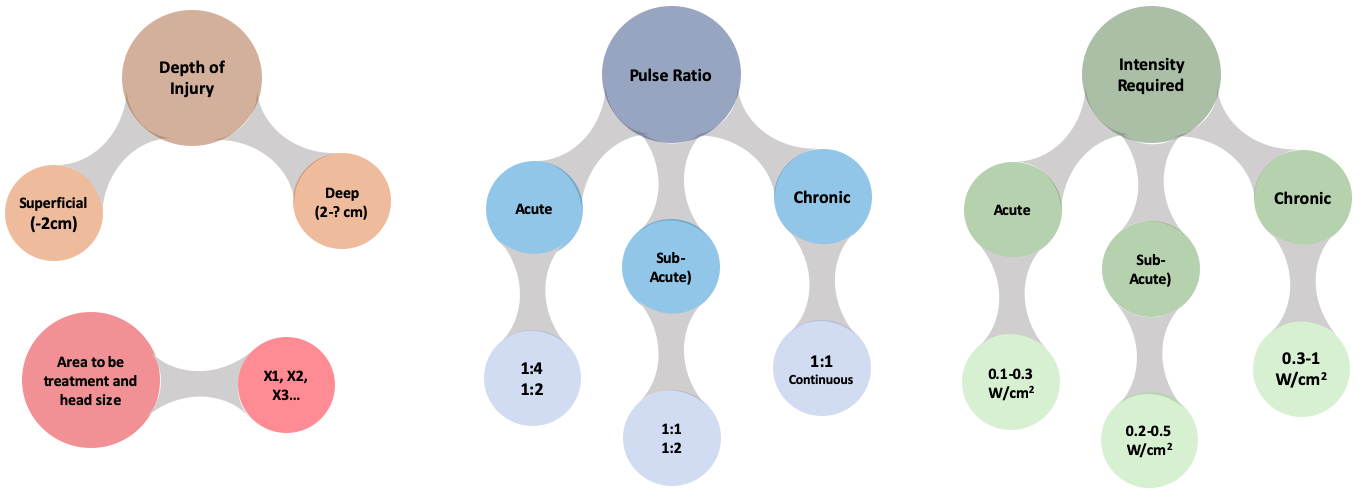
Figure 6. Ultrasound dose calculation based on depth of injury, pulse ratio, intensity required and area to be treated based on Watson et al. 9,13
Source: Author´s own preparation
New dosimetry parameters
Effective radiating area (ERA): is the area of a therapeutic ultrasound head that produces useful ultrasonic energy. It is measured in square centimeters and calculated by the identification of all points where the energy is at least 5% of the maximum measured intensity at the transducer´s surface42 . ERA is always smaller than the transducer surface and it may vary depending on the manufacturer and treatment area should not exceed 2-3 times ERA (see figure 7.a).
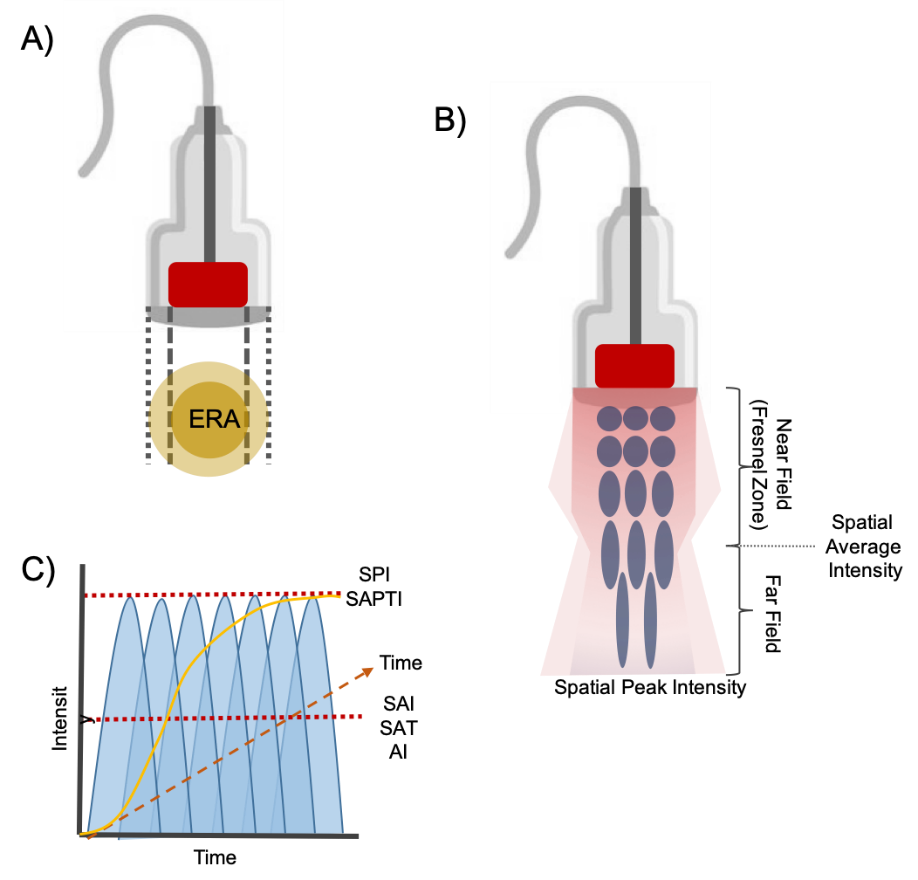
Figure 7. New approach to therapeutic ultrasound dosimetry parameters. A) effective radiating area, B) beam profile, C) spatial average- temporal average/peak intensity.
Source: Author´s own preparation
Beam non-uniformity ratio (BNR) : refers to the ratio between spatial-peak and spatial average intensities within the ultrasound beam43. A perfect beam must have a relation of 1:1. It describes the amount of variation in the beam, minimally acceptable is 8:1. The actual peak output is equal to the SAI* BNR.
Spatial average intensity (SAI) : It is the most used dose-determining parameter among clinicians. This measure is assessed in W/cm2 and it is obtained by dividing the experimental power (total power) by the experimental ERA 42 (see figure 7.b). If 20 W being applied with a 10cm2 ERA resulting in 1.5W/cm2.
Spatial peak intensity (SPI): the energy is uniform as it gets closer to the middle of the transducer head, this is called near zone or Fresnel Zone. The beam becomes less consistent farther away from the head, that’s where spatial peak intensity is found. The point of maximum acoustical intensity is located between the near and the far zone also known as spatial average intensity (see figure 7.b).
Spatial Average-Temporal Average Intensity (SATAI): this variable is meaningful only during pulsed output. It could be understood as the SAI during time. This variable is estimated by the following formula 44 :
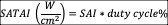
Spatial Average-Temporal Peak Intensity (SAPTI): the average energy delivered during pulsing (on) time of the duty cycle. It could be understood as the SPI during time.
Energy density per treatment : this variable respond to the following equation 44:
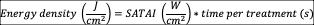
Total energy per treatment: this variable respond to the following equation44:

Total exposure: is the sum of total hours that patient was exposed to an specific treatment, resulting from the following equation44:
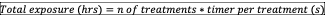
Total energy delivered: is the sum of total energy applied among the treatments, resulting from the following equation44:
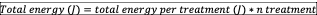
Application speed and movement: some studies have suggest that the movement of the transducer in the application area should be slow (±4cm2/s) and in circles45.
Low intensity pulsed ultrasound (LIPUS): over the last decade, TUS has amplifies its usefulness to the intervention on bone fractures, healing the fracture and increasing the rate fracture heals. The proposed mode is low intensity pulsed ultrasound (LIPUS) and the benefits seem to be significant using low-intensity (<0.1 W/cm2). LIPUS seems to have some effect on calcium turnover46, prostaglandin signaling pathways47, gene regulation and chondrocyte differentiation48. LIPUS in vitro effects are increase in collagen synthesis, intracellular calcium and proteoglycan synthesis, calcium incorporation, collagen and non-collagen proteins synthesis, IGF-I, osteoblast proliferation, TNFa, NO production and length of calcified diaphysis43.
Ultrasound on bone fracture was usually introduced at an intensity ranging from 0.5 to 2 W/cm2 but with certain complications and bone damage, that is why LIPUS uses SATA below 0.1W/cm2, with the most predominant SATA of 0.03 W/cm2. It is usually applied at 1-1.5MHz of frequency using a pulsing regimen of 1:4 (duty factor: 20%). This new LIPUS modality only increases the temperature <1.0°C, reducing tissue damaging43. It is essential to point out that LIPUS must be applied using stationary treatment head over the fracture side, this is possible due to the low SATA (0.03 W/cm2) and the low BNR of the ultrasound treatment heads used (BNR <4-6), additionally the spatial-peak intensity is <0.12 W/cm2 and ERA of 3.8 to 5cm2. During fracture repair LIPUS is usually applied daily for 20min43. Despite this very promising results much research remains to be done in vivo to determinate whether LIPUS as a mode to treat fractures49.
Conclusion
Ultrasound is suggested as one of the treatment options available for soft tissue musculoskeletal injuries related to sport practice. Considering some limitation in the TUS equipment’s used as that individual transducer from different manufacturers may differ up to 60% in their ability to heat tissue19,50 and there are some misreported ERA and SAI among almost all ultrasound equipment with an intra manufacturer and inter manufacturer variability of 35% and 61% respectively in the measurement of SAI42; it is essential for therapists to address all methodological and protocolary guidelines in order to obtain better results.
Other clinical local contraindications must be carefully analyzed when choosing TUS as therapy modality as pregnancy, cancer, bleeding tissue, hemophilia and other absolute contraindications. Despite there are no cumulative doses defined for any TUS, therapist need to avoid unwanted bioeffects such as burns and vascular injury due to repeated treatments. Therapeutic ultrasound devices are typically complex and subject to deterioration or failure they should be checked for safety operation and verification of appropriate ultrasonic fields to ensure efficient treatment.
The use of low-frequency therapeutic ultrasound (0.5-1W/cm2) has been raising in recent years. It seems to improve the rate of tissue repair following tendon, skeletal muscle, ligament, and tendon-bone junction injuries51 and promises very good insights for its applications in vivo.
References
- Pino-Ortega J, Rojas-Valverde D, Gómez-Carmona CD, Bastida-Castillo A, Hernández-Belmonte A, García-Rubio J, et al.Impact of Contextual Factors on External Load During a Congested-Fixture Tournament in Elite U'18 Basketball Players. Front Psychol. 2019;10:1100. doi: 10.3389/fpsyg.2019.01100.
- Rojas-Valverde D, Gutiérrez-Vargas R, Rodríguez-Montero A, Pereira LA, Loturco I, Martín-Rodríguez S. Reduced muscle contractile function in elite young soccer players after a short-congested fixture period. Proc Ins Mech Eng P J Sports Eng Technol. 2019;233(2):249-257. doi: 10.1177/1754337118817957
- Dellal A, Lago-Peñas C, Rey E, Chamari K, Orhant E. The effects of a congested fixture period on physical performance, technical activity and injury rate during matches in a professional soccer team. Br J Sports Med. 2013;49(6):390-394. doi:1136/bjsports-2012-091290 (Enlaces a un sitio externo.)
- Rojas-Valverde D, Gómez-Carmona CD, Oliva-Lozano JM, Ibáñez SJ, Pino-Ortega J. Quarter’s external workload demands of basketball referees during a European youth congested-fixture tournament. Int J Perform Anal Sport. 2020;20(3):432–444. doi: 10.1080/24748668.2020.1759299
- Rico-González M, Arcos AL, Rojas-Valverde D, Clemente FM, Pino-Ortega J. A Survey to Assess the Quality of the Data Obtained by Radio-Frequency Technologies and Microelectromechanical Systems to Measure External Workload and Collective Behavior Variables in Team Sports. Sensors (Basel). 2020;20(8):2271. doi: 10.3390/s20082271.
- Verhagen EA, Clarsen B, Bahr R. A peek into the future of sports medicine: the digital revolution has entered our pitch. Br J Sports Med. 2014;48(9):739-40. doi: 10.1136/bjsports-2013-093103.
- Rojas-Valverde D, Gómez-Carmona CD, Gutiérrez-Vargas R, Pino-Ortega J. From big data mining to technical sport reports: the case of inertial measurement units. BMJ Open Sport Exerc Med. 2019;5(1):e000565. doi: 10.1136/bmjsem-2019-000565.
- Rojas-Valverde D, Gutiérrez-Vargas JC, Sánchez-Ureña B. Sport Readaptation: Where Do We Draw the Lines Between Professionals? Front Sports Act Living. 2019;1:62. doi: 10.3389/fspor.2019.00062.
- Watson T. The role of electrotherapy in contemporary physiotherapy practice. Man Ther. 2000;5(3):132-41. doi: 10.1054/math.2000.0363.
- Cameron M. Agentes físicos en rehabilitación.5a ed. España: Elsevier; 2019.
- Mostafa J, Ali Y, Zohre R, Samaneh R. Electromagnetic Fields and Ultrasound Waves in Wound Treatment: A Comparative Review of Therapeutic Outcomes. Biosci Biotechnol Res Asia. 2015;12(1):185–95. doi: 13005/BBRA/1622 (Enlaces a un sitio externo.)
- Baker KG, Robertson VJ, Duck FA. A review of therapeutic ultrasound: biophysical effects. Phys Ther. 2001;81(7):1351–8.
- Watson T. Ultrasound in contemporary physiotherapy practice. Ultrasonics. 2008;48(4):321–9. doi: 10.1016/j.ultras.2008.02.004.
- Nussbaum E.The influence of ultrasound on healing tissues . J Hand Ther. 1998;11(2):140-7. doi: 10.1016/s0894-1130(98)80012-4.
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1.
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med. 2009 Jul 21;6(7):e1000097. doi: 10.1371/journal.pmed.1000097
- Office of Health Assessment and Translation (US). Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration. Maryland: OHAT; 2015.
- Lehmann JF, DeLateur BJ, Warren CG, Stonebridge JS. Heating produced by ultrasound in bone and soft tissue. Arch Phys Med Rehabil. 1967;48(8):397–401.
- Merrick MA, Bernard KD, Devor ST, Williams MJ. Identical 3-MHz ultrasound treatments with different devices produce different intramuscular temperatures. J Orthop Sports Phys Ther. 2003;33(7):379-85. doi: 10.2519/jospt.2003.33.7.379.
- Lehmann JF, Warren CG, Scham SM. Therapeutic heat and cold. Clin Orthop Relat Res. 1974;(99):207-45. doi: 10.1097/00003086-197403000-00028.
- Dyson M, Suckling J. Stimulation of tissue repair by ultrasound: a survey of the mechanisms involved. Physiotherapy. 1978;64(4):105–8.
- Sparrow KJ, Finucane SD, Owen JR, Wayne JS. The effects of low-intensity ultrasound on medial collateral ligament healing in the rabbit model. Am J Sports Med. 2005;33(7):1048–56. doi: 10.1177/0363546504267356.
- Leung MC, Ng GY, Yip KK. Effect of ultrasound on acute inflammation of transected medial collateral ligaments. Arch Phys Med Rehabil. 2004;85(6):963-6. doi: 10.1016/j.apmr.2003.07.018.
- Lorena D, Uchio K, Costa AMA, Desmoulière A. Normal scarring: importance of myofibroblasts. Wound Repair Regen. 2002;10(2):86-92. doi: 10.1046/j.1524-475x.2002.00201.x.
- Hill M, Wernig A, Goldspink G. Muscle satellite (stem) cell activation during local tissue injury and repair. J Anat. 2003;203(1):89-99. doi: 10.1046/j.1469-7580.2003.00195.x.
- Toumi H, Best T. The inflammatory response: friend or enemy for muscle injury? Br J Sports Med. 2003;37(4):284-6. doi: 10.1136/bjsm.37.4.284.
- Fyfe MC, Chahl LA. Mast cell degranulation and increased vascular permeability induced by “therapeutic” ultrasound in the rat ankle joint. Br J Exp Pathol. 1984;65(6):671–6.
- Maxwell L. Therapeutic Ultrasound: Its Effects on the Cellular and Molecular Mechanisms of Inflammation and Repair. Physiother. 1992;78(6):421–6. doi: 10.1016/S0031-9406(10)61528-3
- Mortimer AJ, Dyson M. The effect of therapeutic ultrasound on calcium uptake in fibroblasts. Ultrasound Med Biol. 1988;14(6):499-506. doi: 10.1016/0301-5629(88)90111-1.
- Ramirez A, Schwane JA, McFarland C, Starcher B. The effect of ultrasound on collagen synthesis and fibroblast proliferation in vitro. Med Sci Sports Exerc. 1997;29(3):326-32. doi: 10.1097/00005768-199703000-00007.
- Harvey W, Dyson M, Pond JB, Grahame R. The stimulation of protein synthesis in human fibroblasts by therapeutic ultrasound. Rheumatol Rehabil. 1975;14(4):237. doi: 10.1093/rheumatology/14.4.237.
- Young SR, Dyson M. The effect of therapeutic ultrasound on angiogenesis. Ultrasound Med Biol. 1990;16(3):261-9. doi: 10.1016/0301-5629(90)90005-w.
- Culav EM, Clark CH, Merrilees MJ. Connective tissues: matrix composition and its relevance to physical therapy. Phys Ther. 1999;79(3):308-19.
- Gomez MA, Woo SL, Inoue M, Amiel D, Harwood FL, Kitabayashi L. Medical collateral ligament healing subsequent to different treatment regimens. J Appl Physiol (1985). 1989;66(1):245-52. doi: 10.1152/jappl.1989.66.1.245.
- Yang C, Li Y, Du M, Chen Z. Recent advances in ultrasound-triggered therapy. J Drug Target. 2019;27(1):33-50. doi: 10.1080/1061186X.2018.1464012.
- el-Batouty MF, el-Gindy M, el-Shawaf I, Bassioni N, el-Ghaweet A, el-Emam A. Comparative evaluation of the effects of ultrasonic and ultraviolet irradiation on tissue regeneration. Scand J Rheumatol. 1986;15(4):381–6. doi: 10.3109/03009748609098208.
- Franco de Oliveira R, Pires Oliveira DA, Soares CP.Effect of low-intensity pulsed ultrasound on l929 fibroblasts. Arch Med Sci. 2011;7(2):224–9. doi: 10.5114/aoms.2011.22071.
- Knight K, Draper DO. Therapeutic Modalities: The Art and Science. 2a ed. Philadelphia: Wolters Kluwer; 2013.
- Draper DO, Castel JC, Castel D. Rate of temperature increase in human muscle during 1 MHz and 3 MHz continuous ultrasound. J Orthop Sports Phys Ther. 1995;22(4):142-50. doi: 10.2519/jospt.1995.22.4.142.
- Poltawski L, Watson T. Relative transmissivity of ultrasound coupling agents commonly used by therapists in the UK. Ultrasound Med Biol. 2007;33(1):120-8. doi: 10.1016/j.ultrasmedbio.2006.07.026.
- Machet L, Boucaud A. Phonophoresis: efficiency, mechanisms and skin tolerance. Int J Pharm. 2002;243(1-2):1-15. doi: 10.1016/s0378-5173(02)00299-5.
- Johns LD, Straub SJ, Howard SM. Variability in Effective Radiating Area and Output Power of New Ultrasound Transducers at 3 MHz. J Athl Train. 2007;42(1):22–8.
- Warden SJ. A new direction for ultrasound therapy in sports medicine. Sports Med. 2003;33(2):95-107. doi: 10.2165/00007256-200333020-00002.
- Alexander LD, Gilman DRD, Brown DR, Brown JL, Houghton PE. Exposure to low amounts of ultrasound energy does not improve soft tissue shoulder pathology: a systematic review. Phys Ther. 2010;90(1):14-25. doi: 10.2522/ptj.20080272.
- de Jesus JF, de Albuquerque TAB, Shimba LG, Bryk FF, Cook J, Pinfildi CE.High-energy dose of therapeutic ultrasound in the treatment of patellar tendinopathy: protocol of a randomized placebo-controlled clinical trial. BMC Musculoskelet Disord. 2019;20(1):624. doi: 10.1186/s12891-019-2993-2.
- Warden SJ, Favaloro JM, Bennell KL, McMeeken JM, Ng KW, Zajac JD, et al. Low-intensity pulsed ultrasound stimulates a bone-forming response in UMR-106 cells. Biochem Biophys Res Commun. 2001;286(3):443-50. doi: 10.1006/bbrc.2001.5412.
- Dekel S, Lenthall G, Francis MJ. Release of prostaglandins from bone and muscle after tibial fracture. An experimental study in rabbits. J Bone Joint Surg Br. 1981;63-B(2):185-9. doi: 10.1302/0301-620X.63B2.7217139.
- Chen YJ, Wang CJ, Yang KD, Chang PR, Huang HC, Huang YT, et al. Pertussis toxin-sensitive Galphai protein and ERK-dependent pathways mediate ultrasound promotion of osteogenic transcription in human osteoblasts. FEBS Lett. 2003;554(1-2):154-8. doi: 10.1016/s0014-5793(03)01157-8.
- Della Rocca GJ. The science of ultrasound therapy for fracture healing. Indian J Orthop. 2009;43(2):121-126. doi: 10.4103/0019-5413.50845.
- Holcomb WR, Joyce CJ. A Comparison of Temperature Increases Produced by 2 Commonly Used Ultrasound Units. J Athl Train. 2003;38(1):24–7.
- Best TM, Wilk KE, Moorman CT, Draper DO. Low Intensity Ultrasound for Promoting Soft Tissue Healing: A Systematic Review of the Literature and Medical Technology. Intern Med Rev (Wash DC). 2016;2(11):271. doi: 10.18103/imr.v2i11.271.